Abstract
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) represents several distinct clinical pathologic entities recently identified by molecular profiling. Treatment with anti-CD19 chimeric antigen receptor (CAR) T-cell therapies is now standard for many patients with relapsed/refractory (R/R) disease. Although antigen loss of CD19 represents a known cause of late relapses, the majority of CAR-T cell treatment failure occurs very soon after treatment at which time the impact of molecular subtype and other somatic mutations of DLBCL is undefined. We sought to determine impact of molecular features of DLBCL tumors on clinical outcomes in a cohort of patients with R/R disease who were treated with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) in order to provide insight into the mechanism of response or resistance to CAR-T cell therapy.
METHODS
We collected clinical data and formalin-fixed, paraffin embedded (FFPE) biopsy specimens from 121 DLBCL patients at the time of R/R disease after prior treatment with standard chemoimmunotherapy across 12 US academic medical centers who subsequently received commercial CAR-T cell treatment. Whole exome and transcriptome sequencing was performed on all cases to measure gene expression and gene copy number alterations. Genetic analysis was done on 96 patients with pre-treatment biopsies that passed sequencing quality filters, and expression analysis on 93 patients. Progression-free and overall survival (PFS, OS) measured from the day of CAR-T cell infusion were estimated using the Kaplan-Meier method and compared with the log-rank test.
RESULTS
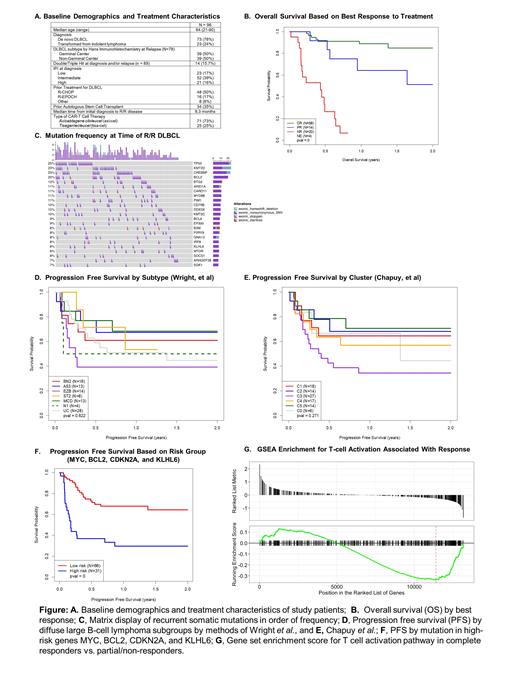
Baseline demographics and treatment details of the patient population are shown in the Table (Panel A). Best overall response was CR in 43% of patients and PR in 10% patients. PFS and OS were significantly different based on best response to treatment (P<0,001 Figure, Panel B). At the time of R/R disease, the most commonly mutated genes were TP53 (25%), KMT2D (23%), CREBBP (23%), BCL2 (20%), BTG2 (12%), ARID1A (11%), CARD11 (11%), MYD88 (11%) and PIM1 (11%), (Panel C). Molecular subtyping based on the method of Wright, et al. revealed cases to be BN2 (N=16), A53 (N=13), EZB (N=14), MCD (N=13), N1 (N=4), ST2 (N=8) and unclassifiable (UC) in 28 cases. Cluster analysis as described by Chapuy et al. assigned cases to be C0 (N=6), C1 (N=18), C2 (N=14), C3 (N=27), C4 (N=17) and C5 (N=14). The impact of subgroups on of PFS are shown in Panels D and E. While not statistically significant different across all groups, there was a trend towards improved outcomes in C5/MCD as well as the C2/A53 subtypes and a trend towards inferior PFS in the C3/EZB subtypes. Inferior PFS was observed in patients with mutations in BCL2 (P=0.009) and MYC (P<0.001), but not BTG2 (P=0.095), MYD88 (P=0.106), or CD79B (P=0.086). An unbiased model comprising mutations in MYC, BCL2, CDKN2A, and KLHL6 was strongly associated with a lack of response to CAR-T therapy and a poor prognosis (HR=3.55, P<0.001, Panel F). Gene Set Enrichment Analysis (GSEA) identified a gene signature reflecting T-cell activation in the pre-treatment tumor biopsy as being associated with a higher likelihood of response to treatment (Panel G).
CONCLUSIONS
DLBCL patients whose tumors have molecular features that are predictive of inferior response to standard frontline treatment including the high-risk subgroups (C2/A53) and (C5/MCD) have favorable treatment outcomes with CAR-T cell therapy. In contrast, individual driver mutations including MYC and BCL2, CDKN2A, and KLHL6 are associated with inferior PFS with CAR-T cell therapy, while mutations in BTG2, MYD88, and CD79B are associated with a favorable PFS. In addition, gene expression analysis implicates a potential role for the microenvironment in modulating responses to CAR-T therapy. These findings suggest that predictive biomarkers for response to traditional chemoimmunotherapy and cellular immunotherapy are distinct. Our results provide insight into potentially targetable pathways for the development of rational treatment strategies that may augment response CAR-T cell therapy.
Hill: Celgene (BMS): Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Gentenech: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Incyte/Morphysis: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. McKinney: Novartis: Research Funding; Nordic Nanovector: Research Funding; Molecular Templates: Consultancy, Research Funding; Kite/Gilead: Honoraria, Speakers Bureau; Incyte: Research Funding; Genetech: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy; Celgene: Consultancy, Research Funding; BTG: Consultancy; Beigene: Research Funding; ADC Therapeutics: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy; Verastem: Consultancy. Neff: Spring Discovery: Consultancy, Ended employment in the past 24 months; EUSA Pharma: Speakers Bureau; Enzyvant: Consultancy. Reshef: BMS, Regeneron, TScan, Synthekine, Atara, Jasper, Bayer: Consultancy; ilead, BMS, Precision, Immatics, Atara, Takeda, Shire, Pharmacyclics, Incyte: Research Funding; Bayer: Consultancy; Gilead and Novartis: Honoraria. Oluwole: Janssen: Consultancy; Pfizer: Consultancy; Curio Science: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding. Ghosh: Incyte: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genmab: Consultancy, Honoraria; Epizyme: Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria; AbbVie: Honoraria, Speakers Bureau; Karyopharma: Consultancy, Honoraria; Genentech: Research Funding. Chen: Actinium Pharmaceuticals: Other: Principal Investigator, SIERRA Trial, Actinium. Hernandez-Ilizaliturri: AbbVie: Other: Advisory Boards; Incyte: Other: Advisory Boards; Celgene: Other: Advisory Boards; BMS: Other: Advisory Boards; Pharmacyclics: Other: Advisory Boards; Amgen: Other: Advisory Boards; Kite: Other: Advisory Boards; Gilead: Other: Advisory Boards; Epyzime: Other: Advisory Boards. Shah: Lily: Consultancy, Honoraria, Research Funding; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Kite: Consultancy; Epizyme: Consultancy; Legend: Consultancy; Incyte: Consultancy; Umoja: Consultancy. Stephens: Epizyme: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; JUNO: Research Funding; Novartis: Research Funding; Abbvie: Consultancy; AstraZeneca: Consultancy; CSL Behring: Consultancy; Celgene: Consultancy; Mingsight: Research Funding; Arqule: Research Funding. Patel: Janssen: Consultancy; Kite Pharma: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy, Speakers Bureau; MEI Pharma: Consultancy; Abbvie: Consultancy; Genentech: Consultancy; Bristol Myers Squibb: Consultancy, Speakers Bureau; BeiGene: Consultancy; Morphosys: Consultancy; Pharmacyclics: Consultancy; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy; Lilly: Consultancy. Pagel: Gilead: Consultancy; Actinium Pharmaceuticals: Consultancy; Kite, a Gilead Company: Consultancy; Incyte/MorphoSys: Consultancy; AstraZeneca: Consultancy; Pharmacyclics/AbbVie: Consultancy; Epizyme: Consultancy; BeiGene: Consultancy; MEI Pharma: Consultancy. Hsi: AbbVie Inc, Eli Lilly: Research Funding. Goy: Genentech/Hoffman la Roche: Research Funding; AbbVie/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Consultancy, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Medscape: Consultancy; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians' Education Resource: Consultancy, Other: Meeting/travel support; Xcenda: Consultancy, Honoraria; Genomic Testing Cooperative: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; LLC(Targeted Oncology): Consultancy; Hoffman la Roche: Consultancy; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Rosewell Park: Consultancy; Infinity/Verastem: Research Funding; MorphoSys: Honoraria, Other; Elsevier PracticeUpdate: Oncology: Consultancy, Honoraria; Xcenda: Consultancy; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Vincerx pharma: Membership on an entity's Board of Directors or advisory committees; OncLive Peer Exchange: Honoraria; COTA (Cancer Outcome Tracking Analysis): Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Elsevier's Practice Update Oncology, Intellisphere, LLC(Targeted Oncology): Consultancy; Incyte: Honoraria; AbbVie/Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Michael J Hennessey Associates INC: Consultancy; Novartis: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding; Karyopharm: Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Phamacyclics: Research Funding; Constellation: Research Funding; Hackensack Meridian Health, Regional Cancer Care Associates/OMI: Current Employment. Ohgami: Stemline Therapeutics: Research Funding. Andreadis: CRISPR Therapeutics: Research Funding; GenMAB: Research Funding; Novartis: Research Funding; Roche: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Epizyme: Honoraria; Incyte: Honoraria; TG Therapeutics: Honoraria; Kite: Honoraria; Karyopharm: Honoraria; Atara: Consultancy, Honoraria; BMS: Research Funding; Merck: Research Funding. Thacker: Data Driven Bioscience: Current Employment. Rozzi: Data Driven Bioscience: Current Employment. Parker: Data Driven Bioscience: Current Employment. Happ: Data Driven Bioscience: Current Employment. Dave: Data Driven Bioscience: Current equity holder in publicly-traded company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract